Glossary
Glossary
What is a Vaccine?
We share our daily lives with a variety of invisible microorganisms, including bacteria, viruses, and parasites. There are many types of microorganisms, most of which are not harmful to humans but some have the potential to cause serious diseases. Microorganisms that enter the body and cause diseases are called pathogens. The human body recognizes pathogens as foreign substances and takes all possible measures to protect itself. The mechanism by which the body recognizes and eliminates pathogens is called immunity, and the cells that play a major role in the pathogen’s elimination are called immune cells. However, when pathogens enter the body for the first time, the immune cells are not sufficiently prepared for an appropriate response. To prepare the body for potentially harmful pathogens, biologists and medical doctors have created vaccines.
The true intention of vaccines is to train the body’s immune system to protect itself from infectious diseases.
Vaccination enhances immunity against pathogens. However, the vaccine does not actually cause a disease like a normal infection (natural infection) but rather weakens the virulence or toxic effects of a pathogen, thus boosting immunity in a controlled and safe manner. Vaccines are similar to simulations of natural infections. In this way, if a pathogen enters the body, the immune system will be better prepared to defend itself.
At present, vaccine research for infectious diseases, such as coronavirus, influenza, Ebola, and AIDS, is underway both in Japan and around the world. Of course, scientists are working hard to keep us safe in our daily lives. In the near future, scientists are aiming to develop vaccines for diseases other than infectious diseases, such as cancer and neurological diseases. Generally, vaccine research is a very broad field of study. Vaccines are revered as the miracle of medicine.
The Role of Vaccines
Vaccines are administered to prevent you from contracting a disease or, if you contract a disease, reduce the severity of the symptoms. Vaccines also prevent the spread of diseases within and between populations, and provide indirect protection for those who cannot be vaccinated, through herd immunity. In Japan, everyone is vaccinated at birth. By doing this, we can protect not only our families but also the community, the country, and the world from infectious diseases. The government is calling for routine vaccinations because they want people, who are a country’s greatest assets, to live healthy and happy lives. Unfortunately, some people cannot be vaccinated due to medical reasons, so let us get vaccinated to protect them. Let us build our future using our own hands. Vaccination was the first step.
”All for one and one for all.”
The History of Vaccines
In 1798, Edward Jenner, an English medical practitioner, reported the prevention of smallpox using cowpox (smallpox that affects cattle). To our knowledge, this is the first scientifically documented vaccine in human history. Approximately 100 years later, in the 1880s, Pasteur (France) and Koch (Germany) created the basis for vaccines against microorganisms.
In particular, Pasteur developed the idea of “artificially creating a vaccine that causes a weak disease from one that causes a strong disease,” and developed many principles of vaccines that are still applied today. In the 1900s, as new viruses and bacteria were discovered, the technology to produce vaccines progressed using innovative methods, such as the use of bird eggs to increase the number of virus particles for vaccines, artificial cell cultivation, and recombinant DNA technology.
In addition, the safety of vaccines has improved with the change from classical varicella to live vaccines, and from live vaccines to inactivated vaccines. In 1980, the WHO declared the eradication of smallpox, a disease that had plagued humankind for decades. This was the first example of disease control through vaccination. Polio (acute poliomyelitis) is also on the verge of eradication. Currently, mRNA, peptide, and inactivated vaccines produced by new technologies are being used against new coronaviruses and differ somewhat from conventional vaccines, though their safety and efficacy are still under question. Although it is now possible to obtain vaccines against various fatal infectious diseases, people have great distrust for the technology, even in Japan. Vaccines are not designed to harm people, but to help them.
Many Japanese people, including Shibasaburo Kitasato, the “father of Japanese bacteriology,” were deeply involved in the development of vaccines. In Japan, the idea of vaccines was adopted in the latter half of the Edo Shogunate period of national isolation, with desperate Japanese doctors employing the knowledge produced by Dutch scholars (as written in “Snow Flower”). This is a word from the president of the academic meeting of the Japanese Society for Vaccine Research
Vaccine preventable diseases (VPD)
Diseases that can be prevented by vaccines are called “VPD”.
Vaccine
Preventable
Diseases
During infancy, the body’s resistance to disease is not fully developed, and is more susceptible to a variety of infectious diseases. Infants become immune to these diseases through eventual infection. However, some infections, such as measles and polio, can cause serious symptoms and sequelae. Therefore, it is necessary to prevent infectious diseases before they occur. Additionally, vaccinations are thought to be the safest and most reliable way to protect children from infectious diseases. It is difficult to feel the effects of vaccines when you are healthy, but they actually protect our health from severe disease.
However, in Japan, more children are affected by VPDs than in other developed countries. One of the reasons for this is the low vaccination rate. As a social issue, the necessity and safety of vaccination is not properly communicated, and there are many misunderstandings about vaccines, such as their effectiveness and safety. As a political issue, some vaccines are available in other countries but not in Japan, and some vaccines are expensive.
However, the vaccination trend in Japan is gradually changing. For example, vaccines for HPV, the virus that causes cervical cancer, were associated with several concerns regarding adverse reactions. However, HPV vaccine recommendations have resumed, and a more effective 9-valent vaccine was rolled out in March 2021. As a result, Japan is gradually moving toward the eradication of cervical cancer.
Types of Vaccines
1) Live vaccine
A vaccine in which the virulence (toxicity) of a live virus or bacterium has been weakened as much as possible so as not to cause symptoms (the pathogen is used as-is). Since weakened pathogens multiply in the body, symptoms such as fever and rash may occur some time after vaccination. Because immunity is achieved in a state similar to that of natural infection, the effectiveness of the vaccine is easily achieved.
2) Inactivated vaccine
Inactivated vaccines are prepared by heating, adding phenol, treating with formalin, and irradiating with ultraviolet light to eliminate the pathogenicity of the virus or bacterial pathogen. Some vaccines are further processed by extracting their active ingredients. Inactivated vaccines are safe because they do not multiply in the body after inoculation as live vaccines do, but are less effective than live vaccines; therefore, multiple inoculations or additives called adjuvants are required.
3) Toxoids
A vaccine in which not the pathogen (bacteria) but only the bacterial toxin produced by the pathogen is extracted and made nontoxic by formalin treatment . It is free of poisonous toxins, while maintaining the ability to produce immunity. Similar to inactivated vaccines, multiple doses are required.
The global pandemic caused by the new coronavirus (SARS-CoV-2) discovered in Wuhan, China, in December 2019 triggered the development of a novel type of vaccine. As of 2021, vaccines using genetic information (DNA and RNA) are being developed around the world. Unlike conventional vaccines, the use of genetic information rather than viruses or bacteria has accelerated the development process. In order to prevent another pandemic, the development of vaccines using genetic information will continue to be promoted.
4) mRNA Vaccine
Messenger RNA (mRNA) serve as a blueprint for the production of cellular proteins. mRNA vaccines are formulations that induce appropriate immune responses against the proteins coded for by the mRNA. Since mRNA is easily degraded in the body, it is administered in a stabilized state by encapsulating various modifications of mRNA in lipid nanoparticles that make them less susceptible to degradation. Since the mRNA itself and lipid nanoparticles act as adjuvants, a strong immune response is expected from the mRNA vaccine alone.
The new coronavirus mRNA vaccine is formulated using mRNA that produces the spike protein, which is essential for the new coronavirus to bind to and invade our cells. This spike protein is produced in the body to create immunity against the new coronavirus.
5) DNA vaccine
DNA vaccines are formulated by inserting the DNA required to produce viral and bacterial proteins into a ring-shaped DNA called a plasmid.
Since DNA is synthesized in stages from mRNA to protein, more steps are required to synthesize protein than with mRNA vaccines; however, DNA is more stable and less likely to be degraded in the body.
Plasmids are expected to not only provide immunity through the proteins synthesized from DNA but also activate natural immunity as an adjuvant, resulting in stronger immunity.
6) Virus vector vaccine
This is a recombinant virus preparation in which the DNA required to produce proteins in viruses and bacteria is inserted into a virus vector (carrier) that is harmless to humans. When the virus vector invades cells, it is possible to induce an immune response that mimics viral infection.
Vaccine administration route
In Japan, a vaccine is mainly administered by subcutaneous injection.
Other methods include oral administration, intramuscular injections, and the BCG tube needle method.
1) Subcutaneous administration
In Japan, most vaccines are administered by subcutaneous injection.
The vaccine is administered in the lateral part of the deltoid muscle of the upper arm or in the lower one-third of the posterior side. Infants can also be vaccinated on the anterolateral thigh.
2) Intramuscular administration
Currently, only three vaccines are approved for intramuscular administration in Japan: human papillomavirus (HPV), meningococcal, and 13-valent conjugate pneumococcal vaccines. There are also other vaccines that are supposed to be administered subcutaneously, but some doctors administer inactivated vaccines that contain adjuvants at deeper tissue depths (similar to intramuscular injections) because of strong local reactions. The new corona vaccine manufactured by Pfizer and Moderna, which is currently being circulated, has also been administered intramuscularly (as of May 2021).
3) Transdermal, intranasal, and oral administration
BCG is administered using the tube-needle method (stamp method, Hanko injection), in which a stamp is pressed in two places on the upper arm. Outside of Japan, this is a common practice worldwide.
Rotavirus vaccine was administered orally. Intranasal spray administration has not yet been approved in Japan but is used for live influenza vaccines in Europe and the United States.
Vaccinations
There are two types of vaccines available in Japan: routine vaccination, which is required by law, and voluntary vaccination. Both types of vaccination are recognized for their effectiveness and safety.
1) Routine vaccination
Vaccinations are strongly recommended by national and local governments.
Vaccinations are free of charge if administered at a specified age and during a specified period (based on law).
Routine vaccinations are divided into two categories: vaccinations for infants (obligatory effort) and influenza vaccinations for older adults (non-obligatory effort).
2) Voluntary vaccination
Although it is up to the decision of the person being vaccinated (the guardian in the case of infants), this does not mean that they do not have to be vaccinated. For example, mumps, which is classified as a voluntary vaccination, is covered by routine vaccinations in other industrialized countries due to its sever symptoms, which includes hearing loss in one of every thousand people infected.
Voluntary vaccination is paid for, and since it is not a treatment for a disease, health insurance does not cover it; therefore, in principle, you have to pay for it yourself. However, some local governments provide subsidies at public expenses. Please consult with your local government or doctor before vaccination.
Vaccine situation in Japan and overseas
Japan is a latecomer to the vaccine market, and vaccines that were approved and used in other countries more than ten years ago have been left unapproved. This lack of progress is believed to be due to fears of serious health problems after vaccination. In recent years, the delay in Japan’s vaccine approval compared to other countries (the “vaccine lag”) has gradually improved with the revision of vaccination laws; however, there are still many issues. In the case of the new coronavirus vaccine, the differences in vaccine development, approval, and the time required for vaccination between Japan and other countries (especially Europe and the United States) remain considerable. The following is a list of major issues causing vaccine lags.
Number of vaccinations (routine and voluntary)
Many vaccines are available free in other countries, but are not free of charge in Japan.
For a long time, the WHO has been encouraging routine vaccinations, such as the Hib-, childhood pneumococcal-, and hepatitis B vaccine, which can be given free of charge even in poor countries. These vaccines have only recently become routine in Japan: the Hib vaccine has become available in 2013 (optional in 2008), the pneumococcal vaccine in 2013 (optional in 2010), and the hepatitis B vaccine in 2016 (optional in 1985). Nevertheless, mumps and rotavirus vaccines, which are recommended to be made free of charge in developed countries, have not yet been made available for routine administration. In the U.S., influenza vaccines are routinely administered.
Simultaneous and combined vaccinations
As the number of vaccines increases, so does the number of times they need to be administered, increasing the burden on infants and their parents. In addition, infants must be vaccinated as early as possible to develop immunity against many infectious diseases. Therefore, there is a need for simultaneous vaccinations. In Japan, simultaneous vaccinations are tacitly accepted as “possible at the doctor’s discretion,” but the government has not yet provided a clear direction for simultaneous vaccinations. As a result, some hospitals do not allow simultaneous vaccination.
On the other hand, in the U.S., according to the Centers for Disease Control and Prevention (CDC), it is customary for premature infants as young as two months old to be vaccinated with six different vaccines on the same day. As for combination vaccinations, in Japan, the four-in-one vaccine (diphtheria, pertussis, tetanus, and polio) became available in 2012. However, some countries have adopted the 5-in-1 vaccine (US), which adds Hib to the 4-in-1 vaccine, and the 6-in-1 vaccine (Germany and France), which adds hepatitis B.
Vaccination site
In Japan, intramuscular injection was previously suspected to cause muscle contracture, a condition in which the muscles below the thighs are tense/contracted and do not allow the knees to bend. (Currently, it is denied that this is related to the vaccine, as it is caused by the use of antipyretic and antibacterial drugs.) Under current law, subcutaneous vaccination is encouraged.
In other countries, intramuscular injections in the thigh are common. Many inactivated vaccines contain adjuvants, thus intramuscular injection is preferred to reduce local reactions. In addition, intramuscular injection over a wide vaccination site is necessary to promote simultaneous vaccination. The Japan Pediatrics Society issued a statement in 2011 that actively promoted vaccination in the thigh, which the government will hopefully review in the near future.
In addition, Japan has strict regulations regarding vaccination, intervals, and safety, which have resulted in a lower vaccination rate compared to other countries.
*Only three types of vaccines are limited to intramuscular injection: HPV, meningococcal, and pneumococcal vaccines for older adults.
Vaccination Safety
After vaccination, undesirable symptoms that differ from the signs of immunization are called “adverse reactions.” Most adverse reactions are soreness, redness, and swelling at the vaccination site, and low fever and symptoms subside in approximately one to three days; however, in rare cases, serious adverse reactions may occur.
Adverse event: Any symptom or event that occurs after vaccination, regardless of whether it is causally related to the vaccine.
Adverse reaction: An adverse event in which a causal relationship with the vaccine cannot be ruled out.
Regardless of whether it is a live vaccine or inactivated vaccine, there is a certain pattern of adverse reactions depending on the mechanism of occurrence. In the case of allergic symptoms (anaphylaxis) that appear within a very short period of time due to the ingredients contained in the vaccine, skin and respiratory symptoms appear 30 to 60 minutes after vaccination, followed by cardiovascular symptoms. These symptoms require professional treatment. Similarly, allergic reactions to vaccine components can be observed around 24 hours after vaccination. An early immune response (innate immune response) is also observed within a day of vaccination, and local redness, swelling, and fever appear as systemic symptoms. The symptoms of live vaccines are similar to those of natural infections, although the timing of the symptoms varies depending on the vaccine because of the proliferation of pathogens contained in the vaccine.
In Japan, allergic reactions to gelatin contained in vaccines has been a hot topic. Some people may rightly ask, “Why does a vaccine contain gelatin?” Let us look at the common ingredients of vaccines. The main components of a vaccine include not only the elements that produce immunity (e.g., viruses, bacteria, mRNA, and DNA; see types of vaccines for details) and adjuvants to increase the effectiveness of the vaccine, but also sodium chloride (to mimic biological conditions), potassium chloride, and stabilizers (to stabilize the antigen components). In the past, gelatin was used as a stabilizer. However, owing to the risk of allergies, as of FY2020, the only vaccines against infectious diseases that contain gelatin are those against rabies and yellow fever. However, if you still have any concerns regarding allergies, you may want to ask your family doctor.
In Japan, there is a system that provides relief in cases of severe adverse reactions to vaccines.
In the case of routine vaccinations, there is a system called the vaccination health damage relief system that pays medical expenses based on the vaccination law. As long as it is plausible that the damage was caused by the vaccination, compensation can be received. In contrast, voluntary vaccinations are covered by the adverse drug reaction relief system administered by the Pharmaceuticals and Medical Devices Agency (PMDA).
Vaccines are formulated by weakened or inactivated pathogens that train the body’s immune response; therefore, the possibility of adverse reactions cannot be reduced to zero. This does not mean that we should not be vaccinated, but we need to understand both the risks (adverse reactions) and benefits (beneficial effects) of vaccination. Since the risk-benefit ratio depends on the values of each individual, healthcare providers need to provide correct explanations about vaccination. Ultimately, researchers need to develop safe vaccinations with the lowest possible frequency and severity of adverse reactions. Those involved in administration must establish a system that provides adequate compensation for rare adverse reactions.
For more information on relief from routine vaccinations, please see the website for the Ministry of Health, Labor, and Welfare.
Click here for the Ministry of Health, Labor and Welfare website (Vaccination Health Relief System)
http://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou20/kenkouhigai_kyusai/
For information on relief for voluntary vaccinations, please refer to the Pharmaceuticals and Medical Devices Agency (PMDA).
Click here for PMDA HP (Adverse Drug Reaction Relief System)
Vaccines of the Future
Modern vaccines are mostly administered by subcutaneous or intramuscular injection.
However, there are some problems with injectable vaccines, such as the fact that they are painful and can only be administered by medical personnel. Pain is a major problem for children, and in the case of an emergency, such as a major epidemic, it is difficult to administer vaccines immediately and widely if the current method of vaccination is limited to medical personnel. Therefore, it is desirable to develop vaccines that can be administered easily and painlessly.
For example, intranasal, oral, and transdermal vaccines are still at the research stage. Some intranasal and oral vaccines are already in use; however, research is currently underway to make them safer and more effective. Once these vaccines become available, we may be able to vaccinate ourselves.
Vaccines are also being developed for diseases other than infectious diseases caused by viruses and bacteria. For example, vaccines against cancer; Alzheimer’s disease; lifestyle-related diseases, such as diabetes and hypertension; and allergies, such as those from pollen and food. Vaccines are also expected to improve quality of life by reducing the amount of medication prescribed per capita.
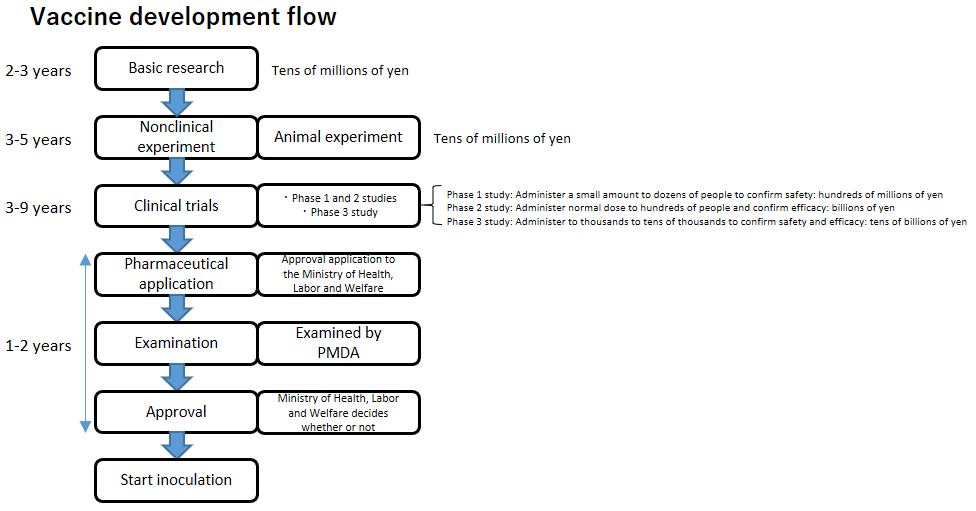
Vaccine Development
What is an Adjuvant?
Adjuvant, from the Latin word “adjuvare” meaning “to help,” is a substance that is administered along with a vaccine to increase its effectiveness (immunogenicity).
Since they are only used to enhance the effectiveness of vaccines, the administration of adjuvants alone will not produce an immune response.
Vaccines in which some components of the antigen are purified are generally less effective; therefore, addition of an adjuvant is necessary.
Addition of an adjuvant activates immune cells that present antigens. Simultaneous administration of adjuvants with vaccines activates both natural and acquired immunity, producing specific antibodies against pathogens and preventing infections.
History of Adjuvants
Reports on adjuvants date to the late 19th century. At the beginning of the 20th century, vaccines for smallpox, rabies, and cholera, among others, were weak live vaccines or vaccines using dead bacteria that were sufficiently effective on their own. However, toxoids, which are similar to inactivated vaccines, have low effectiveness. The importance of adjuvants was recognized in the 1920s, when Ramon and Glenny improved the immunogenicity of diphtheria and tetanus toxoids using aluminum hydroxide (commonly known as Aram). The development of adjuvants has rapidly continued since then.
However, it is still unclear why adjuvants were effective, why empirical vaccine development continued, and why new adjuvants were not approved for some time. However, research in immunology and microbiology during the 1990s, especially in the field of innate immunity and dendritic cells, for which the Nobel Prize in Physiology or Medicine was awarded in 2011, became a catalyst, and research on adjuvants was recognized for its achievements, leading to significant progress. As a result, the development of adjuvants, which have been studied empirically, can now be approached scientifically from the molecular to the biological level, and a wide variety of adjuvants are now being developed worldwide.
The Role of Adjuvants
Adjuvants increase the efficacy of vaccines.
In days when live vaccines and similar vaccines were mainly used, adjuvants were not required in principle because the vaccines themselves were sufficiently effective. However, in the case of toxoid vaccines and subunit vaccines, which were introduced in the 1980s and used not the pathogen itself but some of its components as antigens, the vaccine itself is not sufficiently effective and requires the addition of an adjuvant. With regards to vaccine safety, modern vaccine development is shifting toward safe subunit vaccines, and the importance of adjuvants is inevitably increasing.
Adjuvants have several advantages in addition to increasing efficacy. For example, they can reduce the number of antigens in vaccines and the number of vaccinations, and improve the effectiveness of vaccines for newborns and older adults (i.e., individuals with vulnerable immune systems). Because the amount of antigen required can be reduced, more vaccines can be produced, and, if the adjuvant is inexpensive, the amount of antigen can be reduced, which lowers the price of the vaccine.
Vaccine development has recently expanded beyond infectious diseases to include, for example, cancer; Alzheimer’s disease; lifestyle-related diseases; such as diabetes and hypertension; pollen and food allergies; and autoimmune diseases. However, the targets of vaccines for these “non-infectious” diseases are not able to induce an immune response, and their therapeutic effect is low. Adjuvants, which can induce a strong immune response even in such cases, are expected to be “key” in the future of vaccines and immunotherapy.
Addition of adjuvants is required for subunit vaccines. However, mRNA and DNA vaccines do not require the addition of adjuvants, though some DNA vaccines under development include adjuvants. It has also been reported that mRNA-and DNA-encoding viral sequences themselves are recognized as foreign substances and act as adjuvants to activate immunity.
Adjuvants currently used in vaccines
The best-known adjuvant is aluminum salt, which was first used in 1932 in diphtheria vaccines, and has since been used in pertussis, tetanus, human papillomavirus (HPV), pneumococcus (PCV13), and hepatitis B. It is still the most widely used adjuvant because of its established production method, low cost, and excellent conservation properties. In Japan, it has been the only adjuvant approved until recently. In 1997, an emulsion-type adjuvant containing squalene (an ingredient found in liver oil), MF59, was introduced in Europe as an adjuvant for influenza vaccines.
In Japan, it received approval and was imported in 2009 as a vaccine against the influenza pandemic, which could have an enormous impact on many people, and received special approval. AS04, an improved aluminum salt adjuvant, and AS03, an improved squalene adjuvant, are currently used overseas for HPV and influenza vaccines, respectively. In Japan, AS04 is included in the bivalent HPV vaccine approved in 2013, and AS03 and MF59 have a history of special approval as vaccines against pandemic influenza.
CpG-ODN is currently being considered for use as an adjuvant, and the US FDA has already approved a vaccine against hepatitis B using CpG-ODN. CpG-ODN is part of the DNA of bacteria and viruses, and unlike humans, it is not chemically modified; therefore, it is identified by immune cells as a foreign substance and thus activates innate immunity.
New Adjuvants
With recent advances in immunology, it is now possible to design vaccine development based on scientific evidence, with many new adjuvants being reported. In particular, new adjuvants that target the site on immune cells that recognizes foreign substances are called second-generation adjuvants, many of which are being tested in clinical trials. For example, lipoproteins, lipopolysaccharides, flagella, and nucleic acids are pathogenic components that bind to the “Toll-like receptor (TLR),” which is a pathogen sensor. Currently, an HBV vaccine containing adjuvant nucleic acid components has been approved by the US FDA as HEPLISAV-B. A new coronavirus vaccine containing a nucleic acid component adjuvant has been developed by Clover Biopharmaceuticals and is undergoing phase II/III clinical trials (as of May 2021). In addition, many other ingredients are being tested in clinical trials for various diseases.
The development of adjuvant substances that incorporate drug delivery system (DDS) technology to allow the above adjuvants to be recognized by their target cells is also flourishing. Microcapsules (liposomes) are composed of phospholipid components similar to cells, and nanoparticles can be administered as adjuvants on their surface or within the particles, making it possible to trigger immune responses more efficiently and safely; microcapsules are expected to become next-generation adjuvants.
