Identification of B-cell signaling factors essential for immunological memory in infections and vaccines
2021年12月16日
Michelle S. J. Lee (IMS, Univ Tokyo), Cevayir Coban (IMS, Univ Tokyo/IFReC), and their research group identified an unexpected role of Tank-binding kinase-1 (TBK1) as a crucial B cell intrinsic factor for GC formation during infection and vaccination in mice.
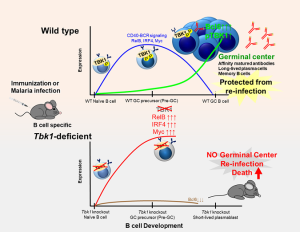
Overview of the effect of TBK1 on activity of other genes and the formation of the germinal center Caption: Normal activity of the enzyme TBK1 in a type of white blood cells called B cells is essential for formation of long-term memory in the immune system. Researchers at UTokyo worked with healthy (Wildtype, top half of image) mice and mice genetically modified to lack TBK1 only in their B cells (TBK1-deficient, bottom half of image). When healthy mice were infected with the mosquito-borne parasite malaria, TBK1 reduces the activity of genes that block B cells’ development, allowing them to grow into mature memory B cells. The B cells of TBK1-deficient mice remain immature, meaning that if the mice survive their first malaria infection, their immune systems retain no long-term memory of the parasite and are extremely vulnerable to a repeated malaria infection. © Michelle S. J. Lee, CC BY-ND-4.0, first published in Journal of Experimental Medicine
Research Background
Current COVID-19 pandemic has brought into our attention that there are several unknown evasion mechanisms by pathogens. To protect host from infection/re-infection, immunological memory must be elevated via one time infection or vaccination. Although this is the main study area of immunology field, however, little is known how immune memory is developed and how long it is durable during infection and vaccination. As an example, malaria infection is one of the most troublesome infections in the world and known that an individual remains susceptible to repeated malaria infection. To date, how malaria infection impairs immune memory that confers sterile protection is not fully understood.
Germinal centers (GC) are highly dynamic structures formed in lymphoid organs and are sites where somatic hypermutation and clonal selection are coupled for high-quality durable antibody responses leading immune memory. The anatomical structure and function of GCs have been studied for a long, but only recently information has been gathered about how B cells are guided through the development of GCs. Upon antigen encounter, B cells rapidly proliferate and differentiate into multipotent precursor cells that have the potential to diverge into two distinct fates: either GC or extrafollicular (EF) responses. Induction of GC reaction is often the desirable outcome of an infection or vaccine to provide antigen-specific long-lasting humoral immunity. Conversely, the induction of GC-independent EF responses in several disease models and vaccinations results in early acute production of short-lived plasmablasts. The intrinsic and extrinsic factors that determine the fate of antigen-activated B cells, whether to enter GC reaction or to commit to EF responses are not yet well-defined.
TANK-binding kinase 1 (TBK1) is a serine/threonine kinase that is famously known for its role in innate immunity especially for type I interferon (IFN-I) production upon nucleic acid recognition. Apart from its role for IFN-I production via phosphorylation of IRF3/7, TBK1 is also involved in various signaling pathways in a cell-type specific manner. Earlier studies found that TBK1 is among one of the genes upregulated in GC B cells upon NP-CGG immunization, suggesting that TBK1 may have a role in GC formation. However, whether TBK1 plays a critical role in GC-dependent memory responses have remained un-answered.
Outline of research results
Here, using both malaria infection and immunization models, we identified TBK1 as a novel B cell intrinsic factor that is crucial in regulating GC differentiation. We found that phosphorylation of TBK1 increased in B cells during GC differentiation (Figure). Notably, TBK1 deletion in B cells led to impaired GC formation and therefore increased susceptibility to a non-lethal malaria infection. Memory B cells generated in the absence of TBK1-dependent GC failed to confer sterile immunity upon re-infection challenge. Although TBK1 deficient B cells could be activated to differentiate into GC precursor cells (Pre-GC), the fate of these cells was distinct from that of TBK1 sufficient B cells. We found that TBK1 negatively regulates both CD40 and BCR signaling in Pre-GC.
Mechanistically, increased CD40-induced RelB expression in Tbk1-deficient Pre-GC enhanced IRF4 expression that subsequently suppressed Bcl6 expression, the master regulator of GC formation. Unlike naïve B cells, BCR activation further enhanced CD40-induced IRF4 expression in Pre-GC in a TBK1-dependent manner. This study revealed a crucial role of B cell intrinsic TBK1 as a determinant of GC commitment through the fine tuning of CD40 and BCR signaling (Figure).
Future prospects
Overall, our findings are expected to have crucial implications for tailoring successful vaccines to produce long lasting protective immunity against several problematic infections like malaria as well as a countermeasure to future pandemics.
TBK1 is required for IFN-I mediated antiviral immunity, while here we additionally showed its importance in GC-mediated humoral immunity. However, TBK1 is also involved in the pathogenesis of autoimmune diseases. Moreover, TBK1 inhibitor, such as Amlexanox has been used in clinical treatment of asthma and recently been proposed for the treatment of obesity related metabolic diseases. Our findings suggest that TBK1 inhibitors should be administered with caution, considering its possible effect on humoral immunity in case of infection and vaccination. Moreover, the timing of TBK1 inhibitor administration may be important to target TBK1 in specific cell types during disease progression and to prevent its suppressive effects on vaccine efficacy.
Publication details
Journal:Journal of Experimental Medicine
Title:B cell intrinsic TBK1 is essential for germinal center formation during infection and vaccination in mice
Authors:Michelle S. J. Lee, Takeshi Inoue, Wataru Ise, Julia Matsuo-Dapaah, James B. Wing, Burcu Temizoz, Kouji Kobiyama, Tomoya Hayashi, Ashwini Patil, Shimon Sakaguchi, A. Katharina Simon, Jelena S. Bezbradica, Satoru Nagatoishi, Kouhei Tsumoto, Jun-Ichiro Inoue, Shizuo Akira, Tomohiro Kurosaki, Ken J. Ishii, Cevayir Coban
DOI:10.1084/jem.20211336
URL: https://doi.org/10.1084/jem.20211336
Contact
Cevayir Coban, Professor, Division of Malaria Immunology, The Institute of Medical Science (IMSUT), The University of Tokyo
UTokyo PEOPLE CEVAYIR COBAN
https://www.u-tokyo.ac.jp/focus/en/people/k0001_02819.html
Division of Malaria Immunology
https://www.ims.u-tokyo.ac.jp/malimmun/index.html
AMED Research Program inquiries
Japan Agency for Medical Research and Development (AMED)
Division of Basic Medical Research, Department of Basic Medical Research
https://www.amed.go.jp/en/index.html
